There is a move that follows myeloma patients and their treatment decisions, and from this learn what works and what does not for the many genetic variations which are present in multiple myeloma. One initiative from the MMRF is called COMPASS, which is following 1000 newly diagnosed patients. Celgene has a 3000 patient study called ConnectMM, and a team sponsored by Takeda Pharmaceuticals will follow 5000 patients and is called INSIGHT-MM. These BIG DATA efforts will all be providing great incites into improvements in treatment. However, there is a data base that has been in existence for years which follows transplant patients both allo and auto, called the CIMBCR(Center for International Blood and Marrow Transplant Research).
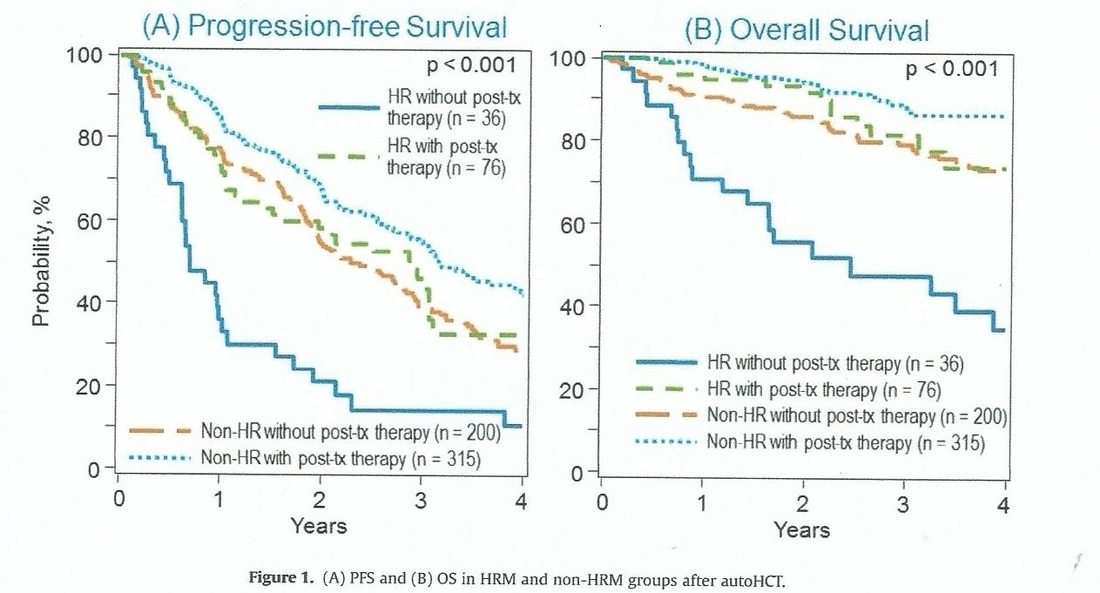
Recently, members of the CIMBCR conducted a retrospective study of high and low risk patients, and obtained some outstanding incites into improved survival for patients with high risk genetic features. Between 2008 and 2012, 715 patients with multiple myeloma identified by FISH and/or cytogenetic data with upfront auto, HCT were identified in the Center for International Blood and Marrow Transplant Research database. HRM(high risk myeloma) was defined as del17p, t(4;14), t(14;16), hypodiploidy (<45 chromosomes excluding -Y) or chromosome 1p and 1q abnormalities; all others were non-HRM. The most significant finding was that HRM patients who had post transplant consolidation or maintenance had a 3 year overall survival rate of 81% versus just 48% for those without post transplant treatment. So HRM patients are 2.7 times more likely to die in three years if they do not have post transplant treatment. You can view the study if you CLICK HERE.
Below you can view this in graphic form.
Good luck and may God Bless your Cancer Journey. For more information on multiple myeloma survival rates and treatments CLICK HERE and you can follow me on twitter at: https://twitter.com/grpetersen1










 RSS Feed
RSS Feed